In organic chemistry, an alkane, or paraffin (a historical name that also has other meanings), is a saturated hydrocarbon. Alkanes consist only of hydrogen and carbon atoms and all bonds are single bonds.[1] Alkanes (technically, always acyclic or open-chain compounds) have the general chemical formula CnH2n+2. For example, methane is CH4, in which n=1 (n being the number of carbon atoms). Alkanes belong to a homologous series of organic compounds in which the members differ by a molecular mass of 14.03u (mass of a methylene group, —CH2—, one carbon atom of mass 12.01u, and two hydrogen atoms of mass ≈1.01u each). There are two main commercial sources: petroleum (crude oil)[2] and natural gas.
Each carbon atom has 4 bonds (either C-H or C-C bonds), and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. The number of carbon atoms is used to define the size of the alkane e.g., C2-alkane.
An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms, for example a methyl or ethyl group.
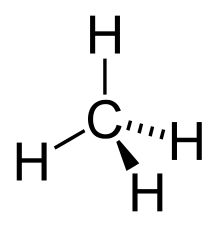
The simplest possible alkane (the parent molecule) is methane, CH4. There is no limit to the number of carbon atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydrocarbon. Waxes include examples of larger alkanes where the number of carbons in the carbon backbone is greater than about 17, above which the compounds are solids at standard ambient temperature and pressure (SATP).
Alkanes are not very reactive and have little biological activity. Alkanes can be viewed as a molecular tree upon which can be hung the more active/reactive functional groups of biological molecules.
Isomerism
Different C4-alkanes and -cycloalkanes (left to right): n-butane and isobutane are the two C4H10 isomers; cyclobutane and methylcyclopropane are the two C4H8 isomers.
Bicyclo[1.1.0]butane is the only C4H6 compound and has no isomer; tetrahedrane (below) is the only C4H4 compound and also has no isomer.
Bicyclo[1.1.0]butane is the only C4H6 compound and has no isomer; tetrahedrane (below) is the only C4H4 compound and also has no isomer.
- C1: methane only
- C2: ethane only
- C3: propane only
- C4: 2 isomers: n-butane and isobutane
- C5: 3 isomers: pentane, isopentane, and neopentane
- C6: 5 isomers: hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane
- C12: 355 isomers
- C32: 27,711,253,769 isomers
- C60: 22,158,734,535,770,411,074,184 isomers, many of which are not stable.

No comments:
Post a Comment